
There’s hope for bladder control issues
While bladder control problems are common, they’re not normal, and often treatable. If you’re one of the tens of millions affected by bladder control problems, you know how these conditions, like overactive bladder (OAB), non-obstructive urinary retention, and fecal incontinence (FI), can interrupt your life. You may have tried changing your diet. Or Kegel exercises and physical therapy. Or medications with unpleasant side effects. But the results just aren’t what you hoped. Don’t give up. You have other choices.
Learn why more than 425,000 patients have used the clinically proven6,7,8 InterStim™ system to help improve bladder and bowel symptoms. Take back control.

Sometimes, medication and physical therapy aren’t enough.
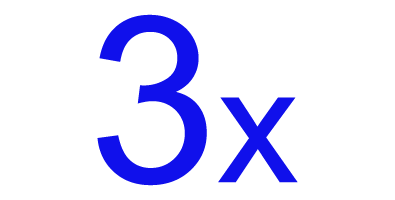
greater improvement in quality of life compared to medications.9†
† Reflects overactive bladder (OAB) patients
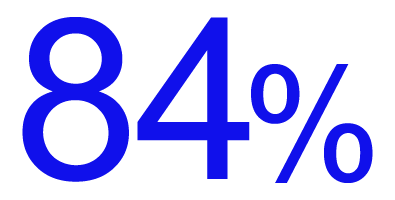
satisfaction among those who use InterStim for bladder control.10
Risks may include pain, infection, lead (thin wire) movement/migration, undesirable changes in urinary or bowel function and uncomfortable stimulation. Talk with your doctor about ways to minimize these risks.
It’s time to speak up and get help!
Get relief from urgent leaks
your louisville bladder symptom specialists
Dr. Barry Pecha
Urology
Physician
First Urology PSC
3920 Dupont Square South
Suite C
Louisville, KY 40207
Phone: 812-704-1639
Dr. Henry Meiers
Urology
Physician
First Urology PSC
3920 Dupont Square South
Suite C
Louisville, KY 40207
Phone: 502-716-5240
Dr. Terrance Blackford
Urology
Physician
First Urology PSC
5120 Dixie Hwy
Ste 105
Louisville, KY 40216
Phone: 502-400-2015
Dr. Aaron Becker
Urology
Physician
First Urology PSC
3 Audubon Plaza Dr
# L10
Louisville, KY 40217
Phone: 502-716-5240
Dr. C. Jeffery Goodwin
Urology
Physician
First Urology PSC
101 Hospital Blvd
Jeffersonville, IN 47130
Phone: 812-704-5060
Dr. Marjorie Pilkinton
Urogynecology
Physician
Norton Urogynecology Center
4123 Dutchman’s Lane
Suite 307
Louisville, KY 40207
Phone: 502-409-5600
Dr. Sarah Kane
Urogynecology
Physician
Norton Urogynecology Center
4123 Dutchman’s Lane
Suite 307
Louisville, KY 40207
Phone: 502-409-5600
Dr. Kellen Choi
Urology
Physician
Urology Associates
731 Hospital Drive, Suite 2
Shelbyville, KY 40065
Phone: 502-405-9450, select option 2 twice
Medtronic provides this listing to assist indicated patients’ access to care. Medtronic has no vested interest in any specific physicians, nor do we provide any recommendation, assurance, or guarantee with respect to their service. Information on this site should not be used as a substitute for talking with your doctor. Always talk with your doctor about diagnosis and treatment information. The list of physicians provided may not include all of the physicians in your area who are qualified to provide care to you. Medtronic does not charge and physicians do not pay to be included on this list. Some physicians on this list may purchase products from Medtronic or may act as paid consultants to Medtronic.
Treatment
You have options
Medtronic Bladder Control Therapy delivered by the InterStim™ systems restores* bladder function by gently stimulating the sacral nerves6,7. It’s sometimes called sacral neuromodulation (SNM). With this therapy, you may experience fewer trips to the bathroom, fewer accidents, and more confidence living with OAB8,9,10. Medtronic Bladder Control Therapy is safe, FDA-approved, and minimally invasive. And it’s been helping people improve their lives for more than 25 years.
*Defined as a 50% or greater reduction in your troublesome bladder symptoms.
Take the first step towards relief.
Request a consultation
"*" indicates required fields
Get back to less worry and more living
Important Safety Information
Medtronic Bladder Control Therapy delivered by the InterStim™ system treats urinary retention (inability to completely empty the bladder) and the symptoms of overactive bladder, including urinary urge incontinence (leakage) and significant symptoms of urgency-frequency. It should be used after you have tried other treatments such as medications and behavioral therapy and they have not worked, or you could not tolerate them. This therapy is not intended for patients with a urinary blockage.
Safety and effectiveness have not been established for pregnancy and delivery; patients under the age of 16; or for patients with neurological disease origins.
Medtronic Bowel Control Therapy delivered by InterStim™ system treats chronic fecal incontinence (an accident or leaking involving stool). It should be used after you have tried other treatments such as medications and dietary modifications and they have not worked, or if you are not a candidate for them.
Safety and effectiveness have not been established for pregnancy and delivery; patients under the age of 18; or for patients with progressive, systemic neurological diseases.
Medtronic Bladder Control Therapy and Medtronic Bowel Control Therapy: You must demonstrate an appropriate response to the evaluation to be a candidate. You cannot have diathermy (deep heat treatment from electromagnetic energy) if you have an InterStim™ device.
In addition to risks related to surgery, complications can include pain at the implant sites, new pain, infection, lead (thin wire) movement/migration, device problems, interactions with certain other devices or diagnostic equipment such as MRI, undesirable changes in urinary or bowel function, and uncomfortable stimulation (sometimes described as a jolting or shocking feeling).
This therapy is not for everyone. This treatment is prescribed by your doctor. Please talk to your doctor to decide whether this therapy is right for you. Your doctor should discuss all potential benefits and risks with you. Although many patients may benefit from the use of this treatment, results may vary. For further information, please call Medtronic at 1-800-328-0810 and/or consult Medtronic’s website at www.medtronic.com.
USA Rx Only. Rev 0517
CITATIONS
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327-336.
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data, 2024. https://www.cdc.gov/nchs/nhanes/Default.aspx. Accessed January 31, 2025.
- US Census Bureau 2020. US adult and under-age-18 populations: 2020 census. https://www.census.gov/library/visualizations/interactive/adult-and-under-the-age-of-18-populations-2020-census.html. Accessed January 31, 2025.
- Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterol. 2009;137(2):512-517.
- Ditah I, Devaki P, Luma HN et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005-2010. Clin Gastroenterol Hepatol. 2014;12:636-643.
- Siegel S, Noblett K, Mangel J, et al. Five-year follow-up results of a prospective, multicenter study of patients with overactive bladder treated with sacral neuromodulation. J Urol. 2018;199(1):229-236.
- Medtronic InterStim Clinical Summary 2018.
- Hull T, Giese C, Wexner SD, et al. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234-245.
- Siegel S, Noblett K, Mangel J, et al. Results of a prospective, randomized, multicenter study evaluating sacral neuromodulation with InterStim™ therapy compared to standard medical therapy at 6-months in subjects with mild symptoms of overactive bladder. Neurourol Urodyn. 2015;34:224-230.
- Foster RT Sr, Anoia EJ, Webster GD, Amundsen CL. In patients undergoing neuromodulation for intractable urge incontinence a reduction in 24-hr pad weight after the initial test stimulation best predicts long-term patient satisfaction. Neurourol Urodyn. 2007;26:213-217.